



The information submited by AFAL can be very useful to characterize the interaction between any ligand and the protein available in PDB database. Using the information about the distance of interaction we can do several studies, and respond some intersting question, for example:
I) Which of the two Nitrogen atoms in histidine is more likely to coordinate a Zn2+ (or Co 2+ or Fe2+) ?
1. Go to the AFAL analysis section and Select Zn as the ligand and choose a distance cut-off of 2.5 Å and submit the job.
2. Once you have some results select the option SHOW ALL in the section "Distance Atoms Residue-Ligand".
3. Copy this information into your text editor (Notepad++) and import the data to any software for Data Analysis and Graphing (Origin, Excel, Gnumeric).
4. Now you can use the filter option to choose only the histidine interaction and then graph the distance between histidine and Zinc atom.
5. You can apply more filter to separate the information corresponding to the atoms of interest (NE2 and ND1).
6. Using the stadistic option or applying the formul of COUNT or FREQUENCY you can know which of the two Nitrogen atoms in histidine is more likley to coordinate a Zn2+, in this case is NE2 with 71% of frequency counts (TABLE)
Atom of His |
Frequency Counts % |
ND1 |
29 |
NE2 |
71 |
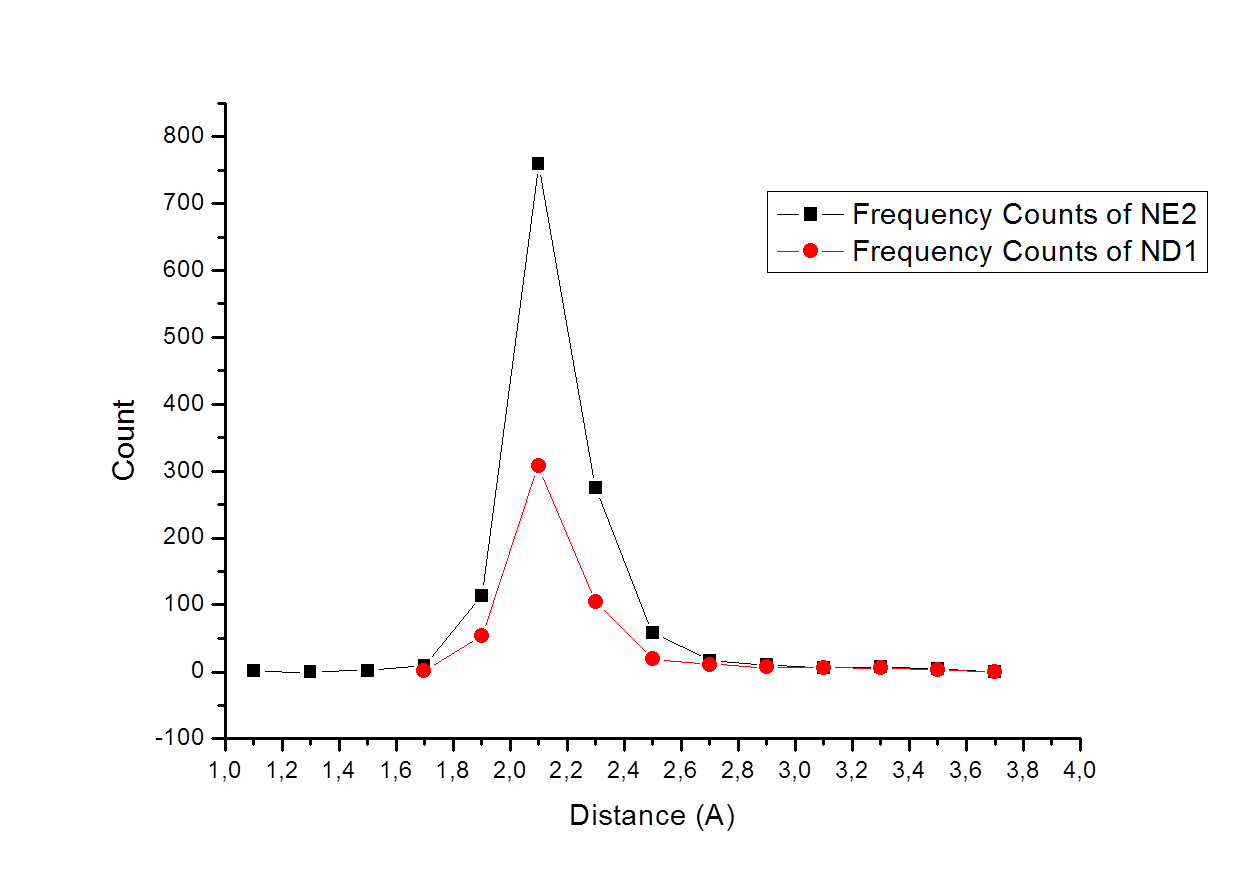
Making a statistical analysis for the data we can obtain graph frequency count vs distance of interaction between Zinc and atom NE2 and ND1 from histidine. We can not observe major differences in the distances between the two possible coordination, la distances mean for the two interaction is 2.1A, but the coordination through the atom NE2 is en general more frequent as we can se in many metalloprotein (Lucarelli, D., et al. J. Biol. Chem, 282(13), 9914–22).
Phosfate group
|
Frequency Counts % |
| alpha | 11 |
| beta | 33 |
| gamma | 56 |
Following the procedure outlined in the previous example was analyzed the binding site for ATP. Analyzing the data delivered by AFAL we can determine that the interaction between the lysine nitrogen (P-loop, Ramakrishnan, et al. Protein Engineering, 15(10), 783–798) occurs most frequently with phosphate gamma range of 56% and 33% for phosphate beta.
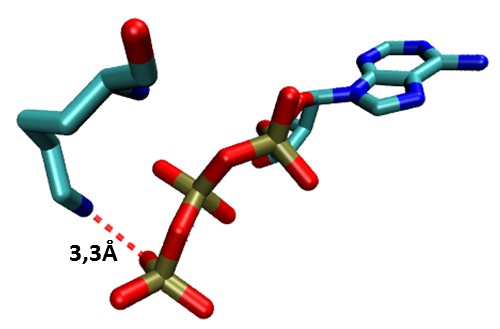
The analysis of distances indicates that the mean for the interaction between the nitrogen of lysine and phosphates alpha, beta and gamma form ATP is 3.3A, with a minimum distance of 2.7A.